In pivotal studies,
LINZESS demonstrated relief of both constipation and abdominal pain in adult patients with IBS-C1
LINZESS was studied in over 1,600 adult IBS-C patients in 2 double-blind, placebo-controlled, randomized, multicenter phase 3 trials. Trials 1 and 2 measured patients who experienced improved CSBM frequency, patients who experienced reduced abdominal pain, and patients who experienced both (combined responders) for at least 6 of 12 weeks and 9 of 12 weeks.
Significantly more LINZESS-treated adult patients were combined responders vs placebo1
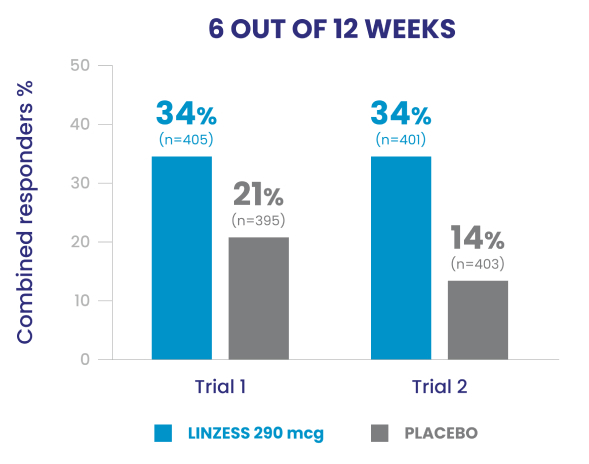
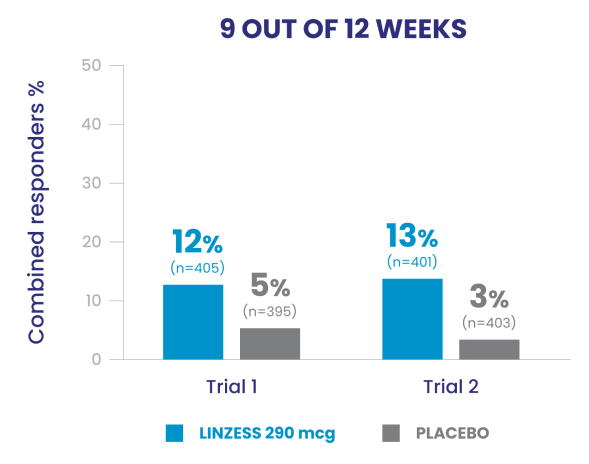
Adult patients with IBS-C had demonstrated improvement in both abdominal pain and frequency of CSBMs1
Improved CSBM frequency1
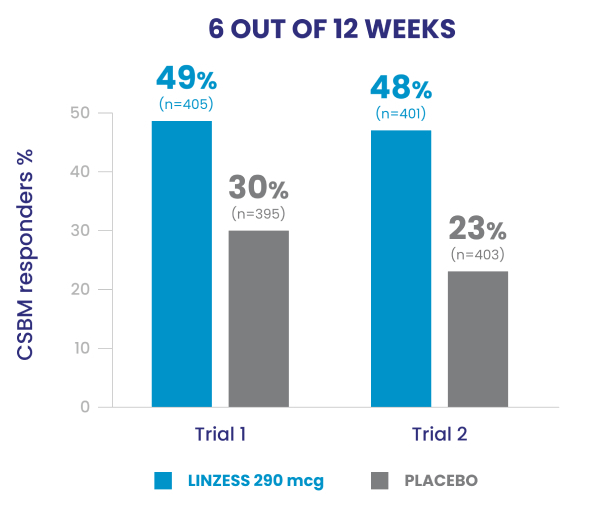
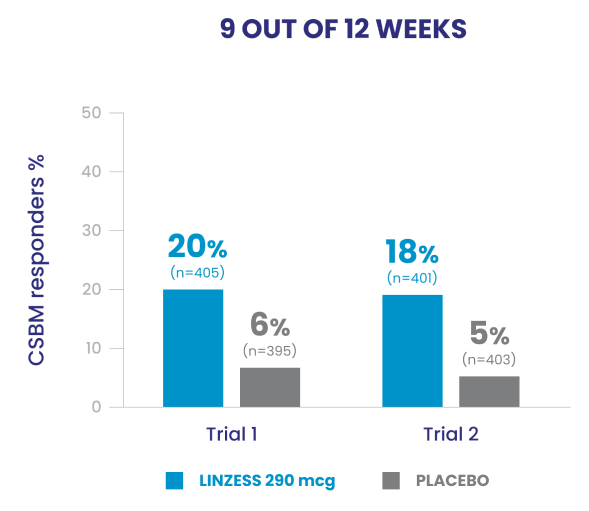
Reduced abdominal pain1
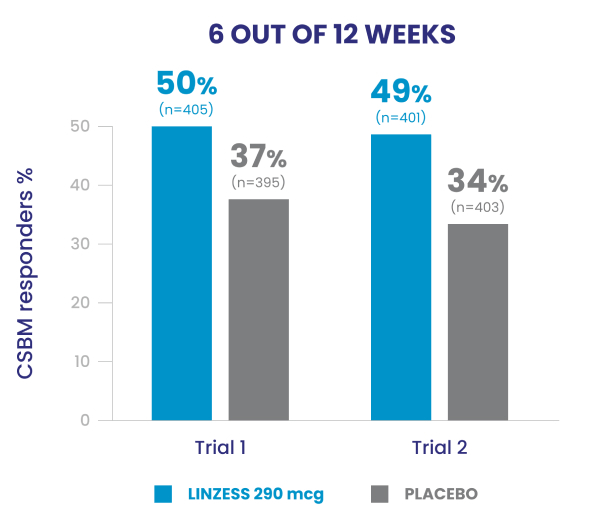
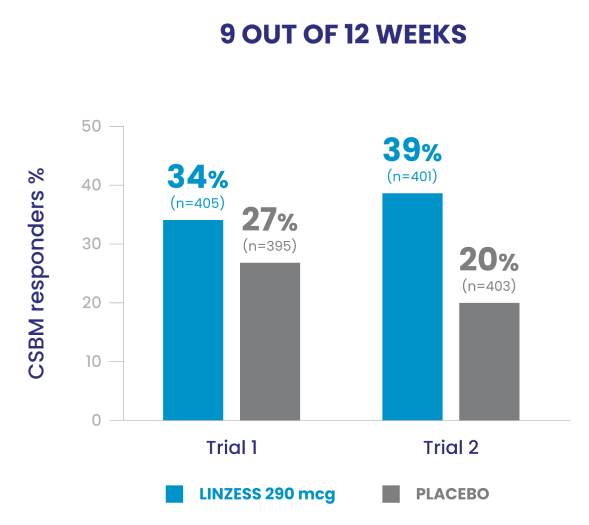
In the IBS-C trials, significantly more patients treated with LINZESS were combined responders than placebo-treated patients. For the 6 out of 12 weeks combined responder endpoint, a patient had to have at least a 30% reduction from baseline in mean abdominal pain and an increase of at least 1 CSBM from baseline, all in the same week, for at least 6 out of 12 weeks. For the 9 out of 12 weeks combined responder endpoint, a patient had to have at least a 30% reduction from baseline in mean abdominal pain, at least 3 CSBMs, and an increase of at least 1 CSBM from baseline, all in the same week, for at least 9 out of the first 12 weeks of treatment.1
Clinical trial design1
Two phase 3 clinical trials (Trial 1 and Trial 2) compared LINZESS 290 mcg vs placebo
- In Trials 1 and 2, the abdominal pain responder, CSBM* responder, and combined responder endpoints were based on a patient being a weekly responder for either at least 9 out of the first 12 weeks of treatment or at least 6 out of the first 12 weeks of treatment
- Trial designs were identical for the first 12 weeks. Thereafter, Trial 1 included a 4-week, randomized withdrawal (RW) period, and Trial 2 continued for 14 additional weeks (total of 26 weeks)
Patients were on average 44 years of age (range, 18-87 years), 90% female, 77% White, 19% Black, and 12% Hispanic
Adult men and women were required to meet Rome II criteria for IBS* and also report during the 2-week pretreatment period:
- Mean abdominal pain score of ≥3 on an 11-point (0-10) numeric rating scale
- <3 CSBMs per week and ≤5 SBMs per week
- Patients who were allowed to continue stable doses of bulk laxatives (eg, fiber) or stool softeners
Patients were not allowed to take osmotic or stimulant laxatives, bismuth, prokinetic agents, or other drugs to treat IBS-C
CSBM, complete spontaneous bowel movement; IBS-C, irritable bowel syndrome with constipation.
*Patients reported for at least 12 weeks (which need not be consecutive), in the preceding 12 months, abdominal discomfort or pain that had ≥2 of these features: relieved with defecation, onset associated with a change in frequency of stool, and/or onset associated with a change in form (appearance) of stool.
In a phase 3b trial, LINZESS improved overall abdominal symptoms (bloating, pain, discomfort) in adult patients with IBS-C1,2
Improvement vs placebo was observed at week 1 and continued through 12 weeks
A 12-week randomized, double-blind, placebo-controlled phase 3b clinical study in adult IBS-C patients (N=614) with a 4-week randomized withdrawal period (posttreatment). Primary endpoint: Change from baseline in the multicomponent abdominal score throughout the treatment period based on the mean abdominal score across treatment weeks obtained using the average daily assessments of abdominal bloating, abdominal pain, and abdominal discomfort. Each abdominal symptom was rated on a 0- to 10-point numeric rating scale where 0=no [symptom] and 10=worst possible [symptom]. Baseline abdominal scores: 6.4 for LINZESS and 6.5 for placebo. The adverse event profile was consistent with the pivotal trials.
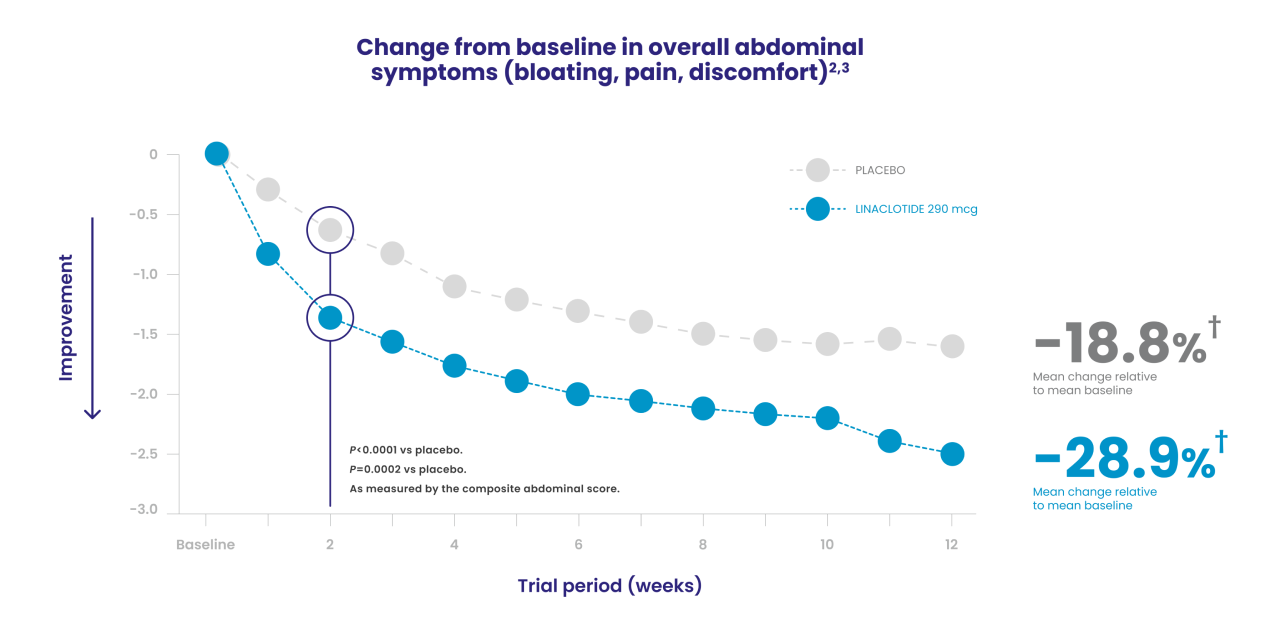
†Abdominal scores at baseline: 6.4 for LINZESS and 6.5 for placebo. Least squares 12-week mean change from baseline: -1.9 for LINZESS and -1.2 for placebo (P<0.0001).1,3
Change from baseline in overall abdominal symptoms (bloating, pain, discomfort) at each week was the multicomponent primary efficacy endpoint.
The adverse event profile was consistent with the pivotal trials.1
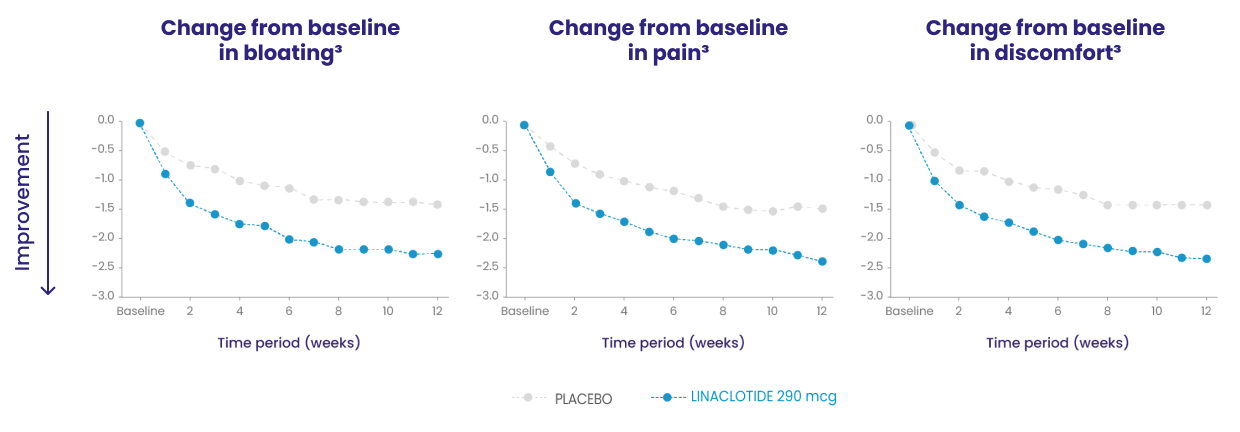
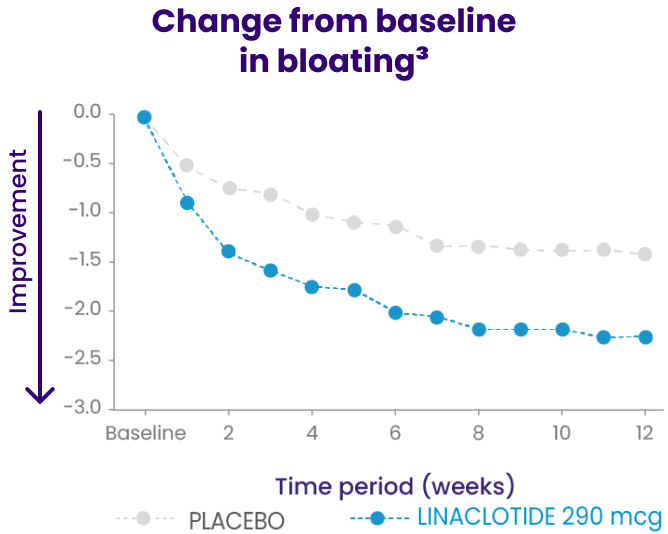
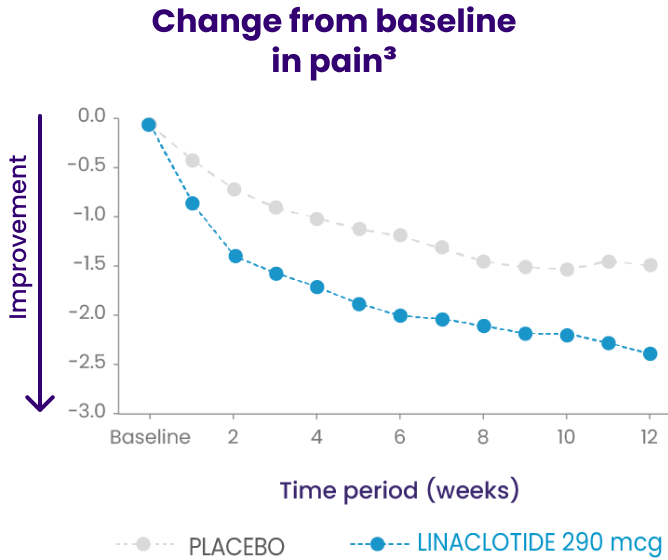
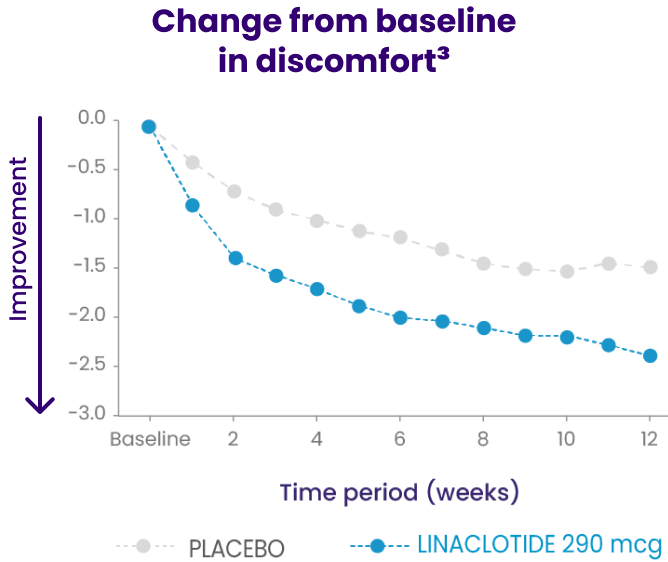
Because these individual component analyses were not adjusted for multiplicity, individual component results need cautious interpretation and could represent chance findings.3
Set expectations with patients on LINZESS
In pivotal studies, adult patients with IBS-C started to feel improvement in CSBM frequency and abdominal pain at week 1 and continued to improve through weeks 6 to 9. Improvement was maintained until the end of the study.1,4
- Diarrhea was the most commonly reported adverse reaction1
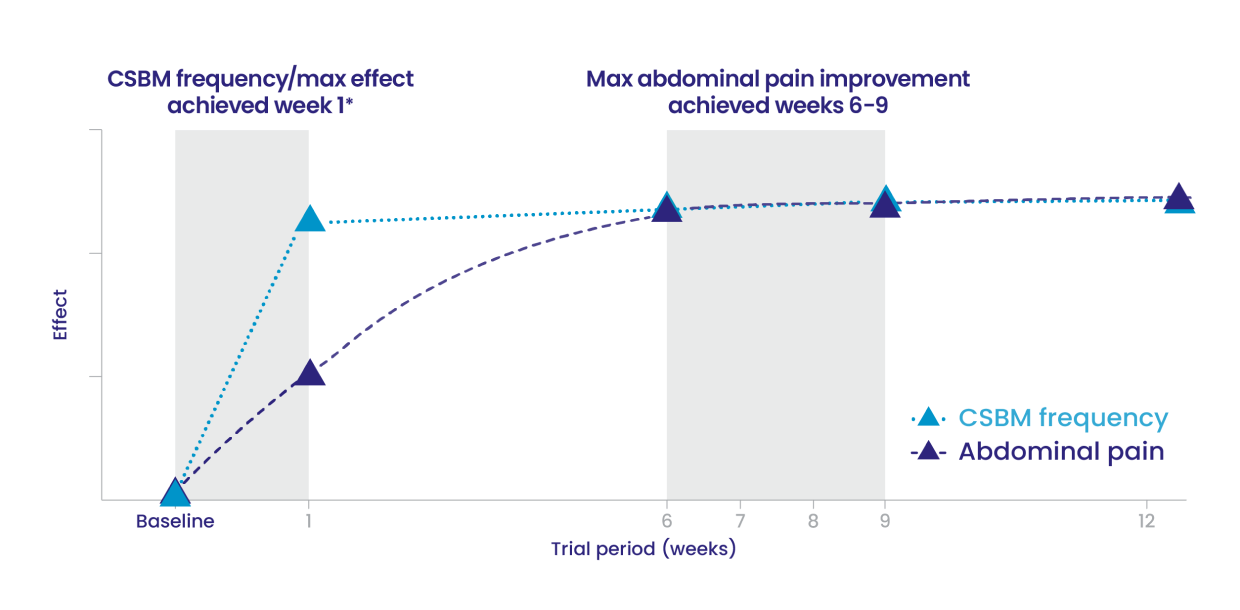
IBS-C Trials 1 and 2: Time to maximum CSBM frequency and abdominal pain improvement with LINZESS treatment1
- This illustrative “line of best fit” graph depicts the time to maximum CSBM frequency and maximum abdominal pain improvement in trials evaluating LINZESS in adults with IBS-C (Trials 1 and 2)
- In each IBS-C trial, maximum effect on CSBM frequency was reached within the first week of treatment with LINZESS and was maintained until the end of the trial
- In each IBS-C trial, for change from baseline in the 11-point abdominal pain scale, LINZESS began to separate from placebo in the first week
- Maximum effect on abdominal pain was seen at weeks 6 to 9 of treatment with LINZESS and was maintained until the end of the study
In clinical trials, patients experienced improvement in abdominal pain and more frequent CSBMs over the 12-week treatment period1,4
- Placebo-treated patients rerandomized to LINZESS experienced an increase of CSBM frequency and a decrease in abdominal pain within 1 week similar to the levels observed in patients taking LINZESS during the treatment period
Patients who continued on LINZESS maintained their response for the additional 4 weeks of the study1,4
- During the 4-week randomized withdrawal period of Trial 1, LINZESS-treated patients rerandomized to placebo experienced a return in CSBM frequency and abdominal pain severity toward baseline within 1 week and did not result in worsening compared to baseline1





