LINZESS is available in a once-daily dose for the following indications1
For IBS-C in adults | 290 mcg
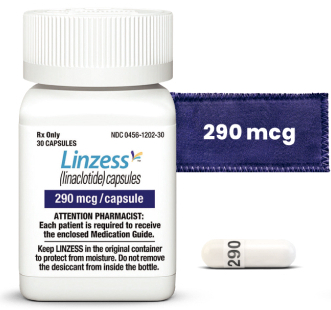
For IBS-C* ICD-10 Diagnosis Code
For CIC in adults | 2 dosing options
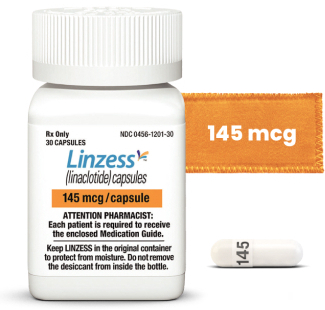
For CIC* ICD-10 Diagnosis Code
For functional constipation in pediatric patients 6 to 17 years of age | 72 mcg
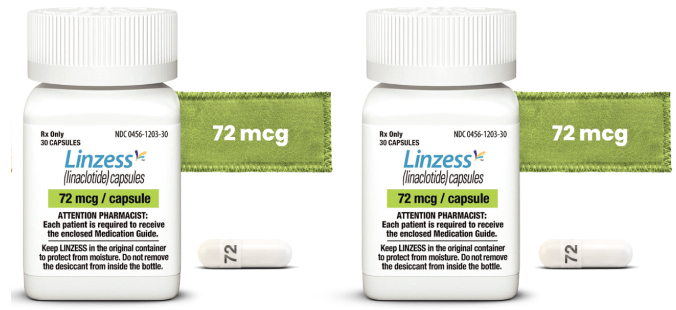
For FC† ICD-10 Diagnosis Code
Capsules shown not actual size. For adults with CIC, a dose of 72 mcg once daily may be used based on individual presentation or tolerability.
For informational purposes only. It is the responsibility of the healthcare provider to determine the appropriate code(s) for services provided to his or her patient. This information does not guarantee payment or coverage. Current as of April 2023.
*For adult patients.
† For pediatric patients 6 to 17 years of age.
CIC, chronic idiopathic constipation; FC, functional constipation; IBS-C, irritable bowel syndrome with constipation; ICD-10, International Classification of Diseases, Tenth Revision.
LINZESS should be taken1:

Every day

Once a day

On an empty stomach and at least 30 minutes prior to a meal

At approximately the same time each day
Clinical considerations1
- Duration of use is not limited
- Dosing adjustments for patients with renal or hepatic impairment are not required
- No drug-drug interactions are anticipated
- Negligible systemic availability




