Well-studied IBS-C safety profile1
LINZESS was evaluated in placebo-controlled trials lasting up to 26 weeks involving more than 2,200 adults with IBS-C
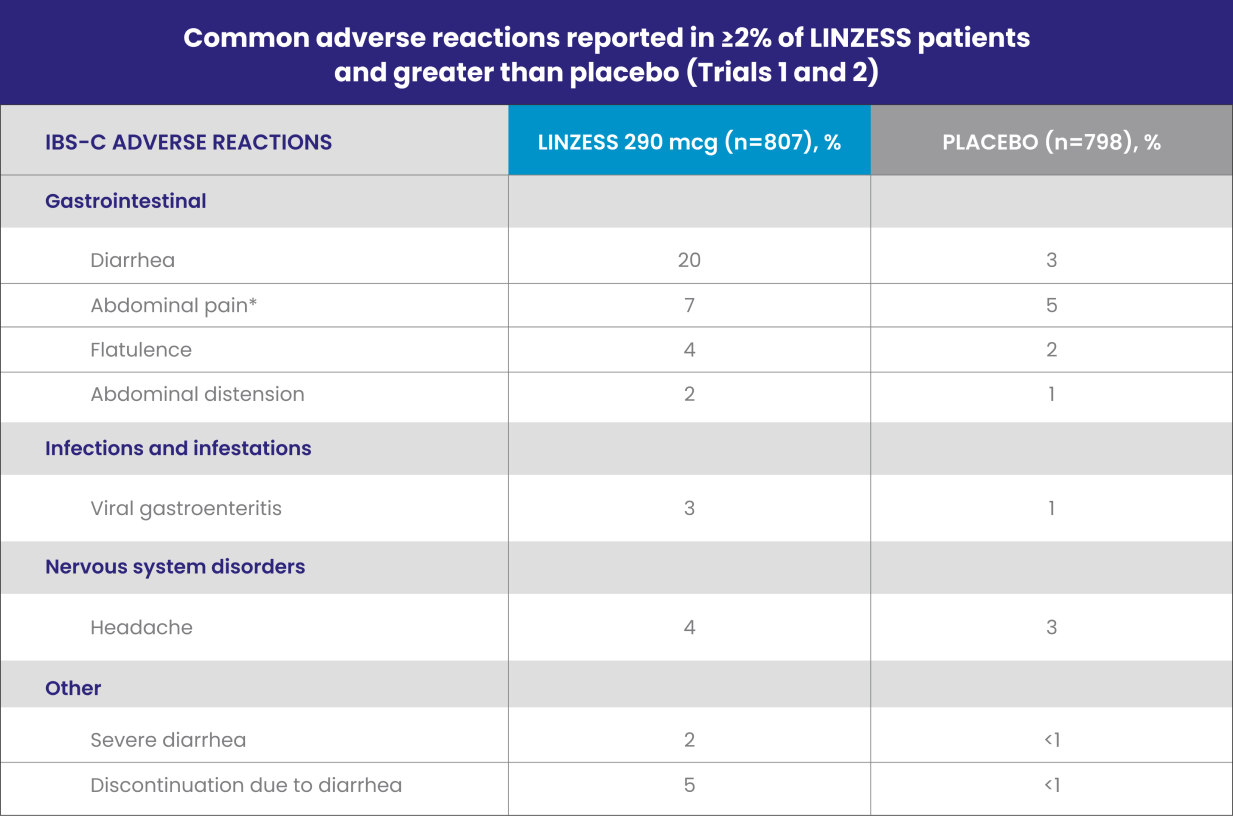
IBS-C, irritable bowel syndrome with constipation.
*“Abdominal pain” term includes the preferred terms: abdominal pain, upper abdominal pain, and lower abdominal pain.
Diarrhea was the most commonly reported adverse reaction of LINZESS1
- In placebo-controlled clinical trials, 2% of the IBS-C patients treated with LINZESS reported severe diarrhea vs less than 1% in patients receiving placebo
- Adverse reactions reported in Trial 6 were similar to those in Trials 1 and 2
LINZESS, a GC-C agonist, is recommended for the treatment of adult patients with IBS-C2,3
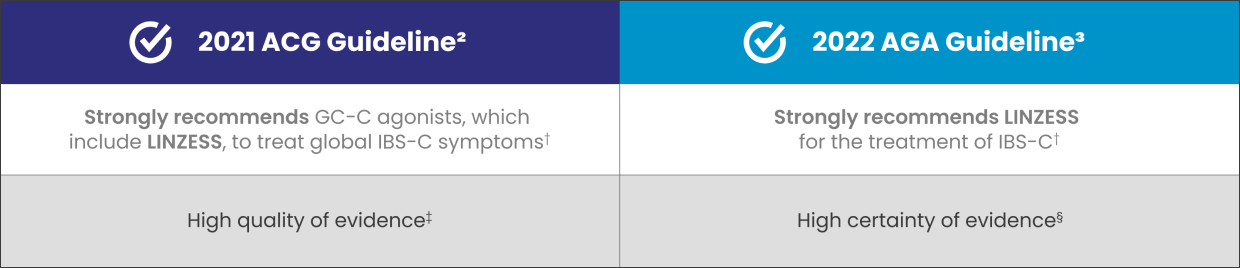
†ACG, a strong recommendation is when most patients should receive the recommended course of action. AGA, for patients, a strong recommendation is when most patients would want the recommended course of action; for HCPs, a strong recommendation is when most patients should receive the intervention. These recommendations are not based on comparative data with other agents.2,3
‡High quality of evidence—the estimate of effect is unlikely to change with new data.2
§High certainty of evidence—very confident that the true effect lies close to that of the estimate of the effect.3
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; GC-C, guanylate cyclase-C; HCP, healthcare professional.



